Sponsored by AbbVie Medical Affairs Intended for Physicians
Chapter 1
An IL-23 Inhibitor’s Phase 3 Clinical Trial Program
Chapter 2
Overview of SEQUENCE, an Open-Label, Head-to-Head Trial Design in Crohn’s Disease
Chapter 3
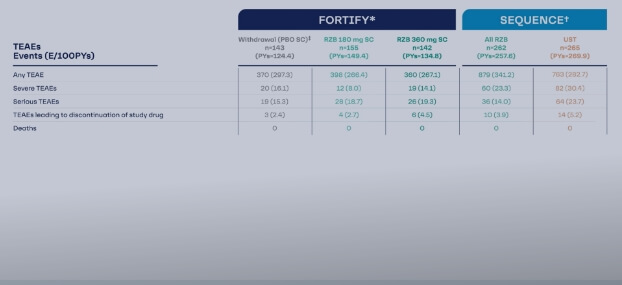
SEQUENCE: Head-to-Head Data from an Open-Label Crohn’s Disease Study
Chapter 4
The Safety Data of an IL-23 Inhibitor in Crohn’s Disease
INDICATIONS
Risankizumab is indicated for the treatment of moderately to severely active ulcerative colitis (UC) in adults.
Risankizumab is indicated for the treatment of moderately to severely active Crohn’s disease (CD) in adults.
IMPORTANT SAFETY CONSIDERATIONS
Risankizumab is contraindicated in patients with a history of serious hypersensitivity reaction to risankizumab or any of the excipients. Serious hypersensitivity reactions, including anaphylaxis, may occur. If a serious hypersensitivity reaction occurs, discontinue risankizumab and initiate appropriate therapy immediately. Risankizumab may increase the risk of infections. Instruct patients to seek medical advice if signs or symptoms of clinically important infection occur. If such an infection develops, discontinue risankizumab until the infection resolves. Evaluate patients for tuberculosis infection prior to initiating treatment with risankizumab. There is a potential for drug-induced liver injury/hepatotoxicity during induction treatment of UC and CD. Monitor liver enzymes and bilirubin at baseline and during induction (12 weeks). Monitor thereafter according to routine patient management. Consider other treatment options in patients with evidence of liver cirrhosis. Interrupt treatment if drug-induced liver injury is suspected, until this diagnosis is excluded. Avoid use of live vaccines in patients treated with risankizumab. The most common adverse reactions (>3%) reported during treatment of UC are arthralgia during induction dosing and arthralgia, pyrexia, injection site reactions, and rash during maintenance dosing. The most common adverse reactions (>3%) reported during treatment for CD are upper respiratory infections, headache, and arthralgia during induction dosing and arthralgia, abdominal pain, injection site reactions, anemia, pyrexia, back pain, arthropathy, and urinary tract infection during maintenance dosing.
Review accompanying risankizumab-rzaa full Prescribing Information for additional information, visit www.rxabbvie.com or contact AbbVie Medical Information at 1-800-633-9110
Chapters
(Subsequent chapters are available upon completion of the prior chapter)
Coming up next in 0:00
Watched
Placeholder
An IL-23 Inhibitor’s Phase 3 Clinical Trial Program
Chapter 1 | 4m 5s

Coming up next in 01:05
Watched
Placeholder
Overview of SEQUENCE, an Open-Label, Head-to-Head Trial Design in Crohn’s Disease
Chapter 2 | 2m 8s
Coming up next in 00:00
Watched
Placeholder
SEQUENCE: Head-to-Head Data from an Open-Label Crohn’s Disease Study
Chapter 3 | 1m 45s

Coming up next in 00:00
Watched
Placeholder
The Safety Data of an IL-23 Inhibitor in Crohn’s Disease
Chapter 4 | 4m 48s
Program Objectives
Evaluate efficacy and show safety results of Risankizumab vs Ustekinumab for the treatment of moderate-to-severe Crohn’s Disease.
Explore the study design and outcomes of Risankizumab’s Phase 3 Crohn’s Disease Program, showing its efficacy across symptomatic and objective endpoints.
Clinical Perspectives Presented by:

Associate Professor at Northwestern University Feinberg School of Medicine

